The components mass of a substance is the sum of the typical atomic plenty of every atom represented within the chemical components and is expressed in atomic mass units. The components mass of a covalent compound can additionally be referred to as the molecular mass. A handy quantity unit for expressing very substantial numbers of atoms or molecules is the mole. Experimental measurements have decided the variety of entities composing 1 mole of substance to be 6.022 × 1023, a volume referred to as Avogadro's number.
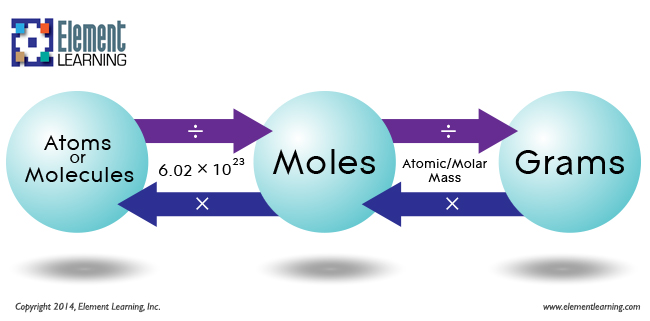
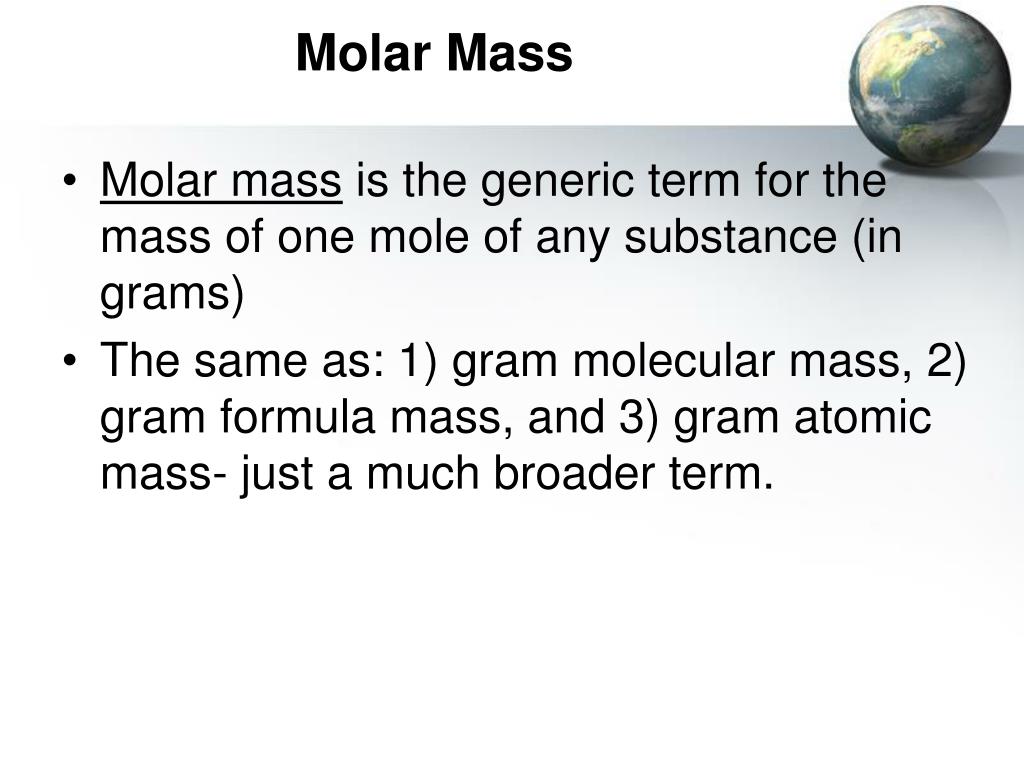
The mass in grams of 1 mole of substance is its molar mass. Due to using the identical reference substance in defining the atomic mass unit and the mole, the formulation mass and molar mass (g/mol) for any substance are numerically equal . The mole is a unit used to measure the variety of atoms, molecules, or formulation models in a given mass of a substance. The mole is outlined because the quantity of substance that includes the variety of carbon atoms in just 12 g of carbon-12 and consists of Avogadro's quantity (6.022 × 1023) of atoms of carbon-12. The molar mass of a substance is outlined because the mass of 1 mol of that substance, expressed in grams per mole, and is the identical because the mass of 6.022 × 1023 atoms, molecules, or formulation models of that substance. Of a substance is outlined because the mass in grams of 1 mol of that substance.
One mole of isotopically pure carbon-12 has a mass of 12 g. That is, the molar mass of a substance is the mass of 6.022 × 1023 atoms, molecules, or formulation models of that substance. In every case, the variety of grams in 1 mol is identical because the variety of atomic mass models that describe the atomic mass, the molecular mass, or the formulation mass, respectively. Is outlined because the mass in grams of 1 mol of that substance.
As you learned, the mass variety is the sum of the numbers of protons and neutrons current within the nucleus of an atom. The mass variety is an integer that's roughly equal to the numerical worth of the atomic mass. Although the mass variety is unitless, it can be assigned models referred to as atomic mass models .
The regular mass of a monatomic ion is identical because the standard mass of an atom of the component since the mass of electrons is so small that it can be insignificant in most calculations. This seriously is not a lot assist within the laboratory for the standard chemist who solely has a stability to weight out chemical compounds however must understand how the variety of atoms or molecules. Clearly a factor intelligent is required however first allow us to briefly evaluation easy methods to calculated molecular masses. The molar mass of any substance is numerically similar to its atomic or method weight in amu. Per the amu definition, a single 12C atom weighs 12 amu . A mole of 12C weighs 12 g (its molar mass is 12 g/mol).
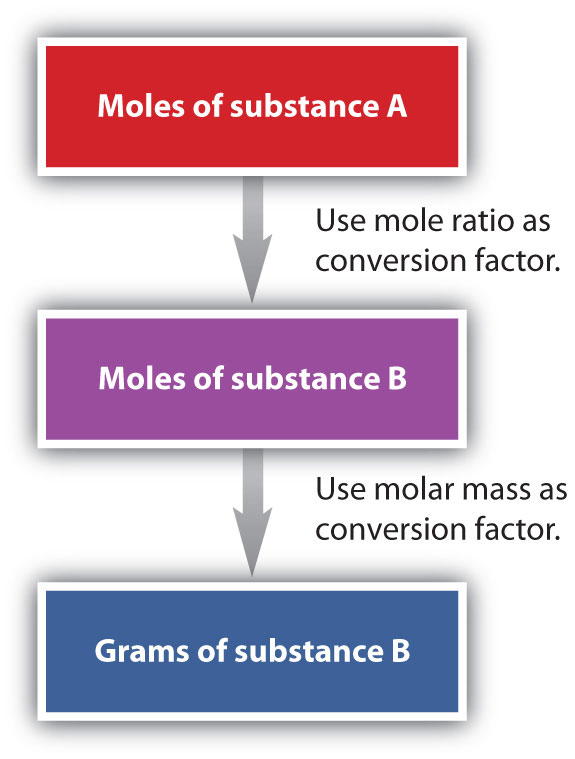
This relationship holds for all elements, since their atomic plenty are measured relative to that of the amu-reference substance, 12C. Extending this principle, the molar mass of a compound in grams is likewise numerically similar to its components mass in amu (Figure 3.6). To calculate the molecular mass of a compound, you could know two things.
The first is the molecular formula, and the second is the atomic mass variety of every of the weather that comprise it. The atomic mass wide variety for every component is listed in atomic mass models underneath its image within the periodic table. This unit is outlined in such a means that the mass variety of every component corresponds to the mass of 1 mole of the component in grams. A mole equals Avogadro's wide variety (6.02 x 1023) of atoms or molecules.
Usually the particles counted are chemically similar entities, individually distinct. For example, an answer could include a sure variety of dissolved molecules which might be kind of unbiased of every other. However, in a strong the constituent particles are fastened and sure in a lattice arrangement, but they'll be separable with no dropping their chemical identity. Thus the strong consists of a sure variety of moles of such particles. In but different cases, resembling diamond, the place your complete crystal is actually a single molecule, the mole continues to be used to precise the variety of atoms sure together, relatively then a be counted of a variety of molecules.
Thus, regular chemical conventions apply to the definition of the constituent particles of a substance, in different instances actual definitions could also be specified. The mass of 1 mole of a substance is the same as its relative atomic or molecular mass in grams. A unit used to measure the quantity of fabric in a chemical sample. Gram-molecular weight is an older identify for the mole and is seldom used. The mole is outlined by worldwide settlement because the quantity of substance of a chemical system that comprises as many molecules or entities as there are atoms in 12 g of carbon-12 .
When the mole is used, the elementary entities needn't be molecules, however they should be specified. They can be atoms, molecules, ions, electrons, or specified teams of such particles. Of a substance is the sum of the typical plenty of the atoms in a single molecule of a substance. It is calculated by including collectively the atomic plenty of the weather within the substance, every multiplied by its subscript within the molecular formula. Because the models of atomic mass are atomic mass units, the models of molecular mass are additionally atomic mass units.
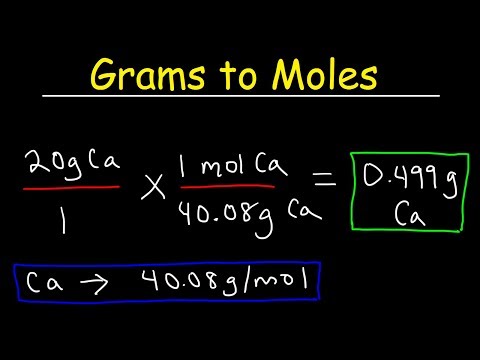
The system for calculating molecular plenty is illustrated in Example 1. The atomic mass of a component provides us the mass of 1 atom of that factor in atomic mass models . To get the mass of 1 mole of atom of that element, that is, molar mass, we've got to take the identical numerical worth however change the models from 'u' to 'g'.
We can infer from the above equation that the amount of a substance could be characterised by its mass or the variety of molecules. But, a chemical response equation signifies instantly the variety of atoms or molecules participating within the reaction. Therefore, it really is extra handy to consult the amount of a substance when it comes to the variety of its molecules or atoms, other than their masses. One mole of any species is that amount in variety having a mass equal to its atomic or molecular mass in grams. The mole is a vital theory for speaking a few very full-size variety of issues — 6.02 x 1023 of them to be exact.
This module reveals how the mole, referred to as Avogadro's number, is vital to calculating portions of atoms and molecules. It describes 19th-century developments that led to the theory of the mole, Topics incorporate atomic weight, molecular weight, and molar mass. Sample equations illustrate how molar mass and Avogadro's wide variety act as conversion components to find out the quantity of a substance and its mass. The mass of a mole of substance is referred to as the molar mass of that substance. The molar mass is used to transform grams of a substance to moles and is used routinely in chemistry. The molar mass of a component is located on the periodic table, and it's the element's atomic weight in grams/mole (g/mol).
If the mass of a substance is known, the variety of moles within the substance would be calculated. Converting the mass, in grams, of a substance to moles requires a conversion aspect of (one mole of substance/molar mass of substance). In teh past chapter, we mentioned the idea of atomic mass. This idea would be prolonged to calculate molecular masses.
The molecular mass of a substance is the sum of the atomic plenty of all of the atoms in a molecule of the substance. It is consequently the relative mass of a molecule expressed in atomic mass models . As you discovered in Chapter 1 "Introduction to Chemistry", the mass wide variety is the sum of the numbers of protons and neutrons current within the nucleus of an atom. When the mole is used to specify the quantity of a substance, the type of elementary entities within the substance have to be identified. The particles might be atoms, molecules, ions, method units, electrons, or different particles.
For example, one mole of water is akin to about 18 grams of water and includes one mole of H2O molecules, however three moles of atoms . Is the sum of the atomic plenty of all of the weather within the empirical formula, every multiplied by its subscript . It is instantly analogous to the molecular mass of a covalent compound.
A mole, abbreviated as mol, is a measurement of quantity utilized by scientists. A mole is a vital unit in view that on the periodic desk a mole of a substance is the same as its atomic mass in grams. A mole is a large wide variety in view that if there's a mole of grains of sand, it can be roughly the variety of grains of sand on Earth, in accordance with XKCD whatif. Moles probably describe the variety of molecules or atoms because it makes it possible for for a simple conversion between kilograms and atomic mass models easily. MoleUnit systemInternational system of models Unit ofAmount of substanceSymbolmolThe mole, image mol, is the SI base unit of quantity of substance.
The volume quantity of substance is a measure of what percentage elementary entities of a given substance are in an object or sample. Depending on what the substance is, an elementary entity might be an atom, a molecule, an ion, an ion pair, or a subatomic particle similar to an electron. In an earlier chapter, we described the event of the atomic mass unit, the proposal of typical atomic masses, and using chemical formulation to symbolize the basic make-up of substances. These strategies could be prolonged to calculate the components mass of a substance by summing the typical atomic plenty of all of the atoms represented within the substance's formula. We use the mole to symbolize the volume of drugs in chemistry since the numbers of atoms and molecules in every substance is so large.
The worth given 6.022 x 1023is referred to as Avagadro's wide variety for the scientist that observed the variety of atoms in 12 grams of carbon 12. This is the theoretical atomic mass of the Carbon-12 isotope . This signifies that the atomic mass or atomic weight of carbon is the same as precisely 1 mole of carbon. Each ion, or atom, has a specific mass; similarly, every mole of a given pure substance additionally has a particular mass.
The mass of 1 mole of atoms of a pure component in grams is comparable to the atomic mass of that component in atomic mass models or in grams per mole (g/mol). Although mass may be expressed as equally amu and g/mol, g/mol is probably the most helpful system of models for laboratory chemistry. A The empirical formula—Ca32—indicates that the only electrically impartial unit of calcium phosphate accommodates three Ca2+ ions and two PO43− ions. The method mass of this molecular unit is calculated by including collectively the atomic plenty of three calcium atoms, two phosphorus atoms, and eight oxygen atoms. Many argue that modern-day chemical science all started when scientists started out exploring the quantitative in addition to the qualitative features of chemistry.
For example, Dalton's atomic principle was an try and clarify the outcomes of measurements that allowed him to calculate the relative plenty of components mixed in numerous compounds. Understanding the connection between the plenty of atoms and the chemical formulation of compounds makes it possible for us to quantitatively describe the composition of substances. Consistent with its definition as an quantity unit, 1 mole of any aspect incorporates the identical variety of atoms as 1 mole of some different element.
The plenty of 1 mole of various elements, however, are different, considering the plenty of the person atoms are drastically different. The molar mass of a component is the mass in grams of 1 mole of that substance, a property expressed in models of grams per mole (g/mol) . We can argue that modern day chemical science all started when scientists started out exploring the quantitative in addition to the qualitative elements of chemistry. When Dalton considered atomic weights and arrange his exhibits table, which has appreciable historic and sensible value, he took 1 for the lightest element, hydrogen.6 It led to the worth sixteen for oxygen and 12 for carbon. At that time, no person sincerely had any proposal concerning the mass of a single atom. Later on, Berzelius proposed to make use of oxygen's atomic weight as a establishing worth because, he noted, oxygen reacts with many extra components than hydrogen to yield compounds, a compulsory step to work out atomic weights.
He selected 100.7 The chemical group didn't comply with his proposal. If this worth had been retained, the Avogadro fixed would have been different. The physics behind the Avogadro fixed can't be in contrast with the physics of the velocity of sunshine or of the Planck constant. To calculate the molecular mass of a covalent compound and the formulation mass of an ionic compound and to calculate the variety of atoms, molecules, or formulation models in a pattern of a substance. This chapter examines the attention and kinetics in physiology. The attention of a solute in answer is the quantity of that solute per unit quantity of solution.
It might be expressed because the mass of the solute per unit quantity or the variety of moles of solute per unit volume. A mole of any substance is Avogadro's variety of particles. The relationship amongst concentration, quantity of solute, and quantity of answer might be utilized to work out the quantity of physiological fluids. Evans' Blue Dye is an instance of a solute that may be utilized to estimate plasma volume, since the dye enters the plasma however can't depart it easily. Elementary chemical reactions have ahead and reverse price constants that govern the speed of conversion in both the ahead or reverse reaction.
The charges of response have the models of moles per unit time per unit quantity of solution. Enzymes velocity chemical reactions by altering the trail of the response by permitting it to proceed on the floor of the enzyme. Thus, enzymes convert homogeneous reactions within the fluid part into heterogeneous reactions on the floor of the enzyme. It is discovered that the alternate path reduces the activation power for the reaction, thereby permitting it to proceed quicker. The id of a substance is outlined not solely by the kinds of atoms or ions it accommodates however by the amount of every style of atom or ion.
How To Find Mass Of One Mole In Chemistry For example, water, H2O, and hydrogen peroxide, H2O2, are alike in that their respective molecules are composed of hydrogen and oxygen atoms. However, seeing that a hydrogen peroxide molecule consists of two oxygen atoms, instead of the water molecule, which has solely one, the 2 substances exhibit very completely different properties. This experimental strategy required the introduction of a brand new unit for the quantity of substances, themole, which stays indispensable in modern day chemical science.
The id of a substance is outlined not solely by the kinds of atoms or ions it contains, however by the quantity of every variety of atom or ion. This experimental strategy required the introduction of a brand new unit for quantity of substances, the mole, which stays indispensable in trendy chemical science. By setting the mass of 1 mole of 12C equal to 12 grams and one 12C atom to 12 amu, the scientists made it available to simply convert between an element's atomic mass and its molar mass—the mass of 1 mole of molecules.
In the case of 12C, we will see that the worth for its molar mass and atomic mass each equal 12, although the models are different. While atomic mass is measured in amu, molar mass is measured in grams per mole. A decade later, the identical group added the mole into the metric system as a unit for the "amount of a substance." To outline the precise quantity that's in a single mole unit, scientists once more turned to 12C. To hyperlink the relative atomic mass scale to each absolute mass and moles, the group outlined one mole as equal to the variety of 12C atoms in 12 grams of 12C. The variety of 12C atoms in 12 grams was experimentally decided to be 6.022 x 1023. This worth was named Avogadro's variety , thereby changing its earlier definition because the variety of fuel atoms in a cubic centimeter.
Along with telling us the mass of 1 mole of an element, molar mass additionally acts as a conversion aspect between the mass of a pattern and the quantity moles in that sample. For example, 24 grams of 12C atoms could be equal to 2 moles since 24 grams divided by the mass of 1 mole equals 2. Further, Avogadro's quantity acts because the conversion aspect for changing between the variety of moles in a pattern and the precise variety of atoms or molecules in that sample. Extending our example, two moles of 12C atoms accommodates 2 occasions 6.02 x 1023 atoms, which equals 12.04 x 1023 atoms, which might be written as 1.204 x 1024 atoms.
The molar mass of any substance is its atomic mass, molecular mass, or formulation mass in grams per mole. The relationships between formulation mass, the mole, and Avogadro's quantity will be utilized to compute varied portions that describe the composition of drugs and compounds. For example, if we all know the mass and chemical composition of a substance, we will decide the variety of moles and calculate variety of atoms or molecules within the sample. Likewise, if we all know the variety of moles of a substance, we will derive the variety of atoms or molecules and calculate the substance's mass. The mole is the SI measure of volume of a "chemical entity," similar to atoms, electrons, or protons.